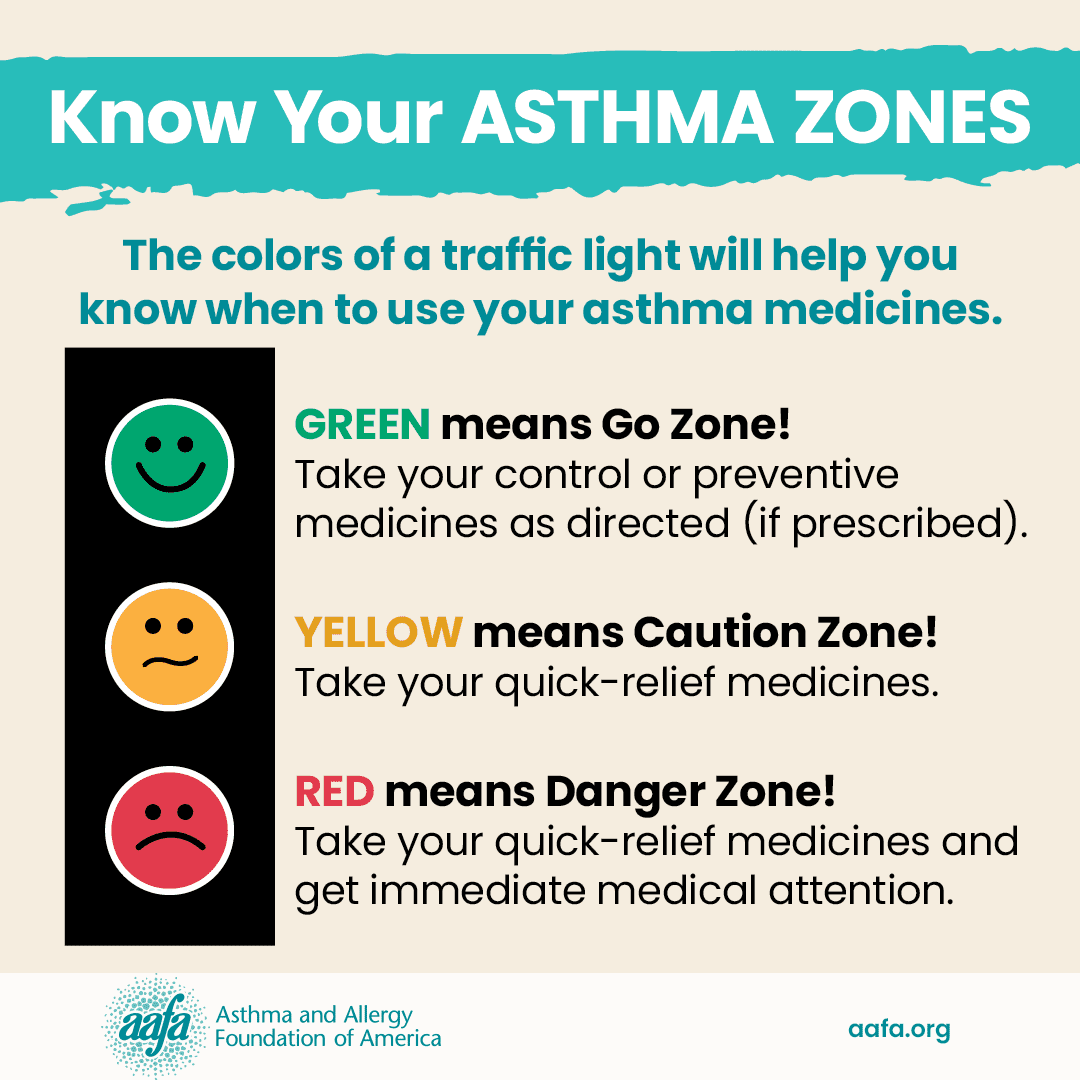
Asthma
Biologics for the Treatment of Asthma
Asthma is a chronic lung disease that causes inflammation (swelling) in your airways. Swollen airways can lead to difficulty breathing, chest tightness, coughing, and wheezing. These are common asthma symptoms. They are usually treated with inhaled medicine and/or pills.
If you have moderate-to-severe asthma, you may have to take routine asthma controller medicine to decrease the swelling in your airways. Sometimes, these medicines are not enough to manage your symptoms. If your routine medicines are not working well on their own, an add-on controller therapy called a biologic might be an option for you.
What Are Biologic Treatments?
Biologic drugs (or biologics) are a kind of medicine that is made by living things, such as animals, plants, or bacteria. Many of these biologics are antibodies, which are proteins that are designed to block specific molecules (tiny particles) in the human body.
Asthma biologics work by disrupting cells or blocking specific molecules that trigger inflammation.
How Do Biologics Treat My Asthma?
Biologics treat your asthma by disrupting the specific cells or blocking specific molecules that make your airways swell after exposure to certain triggers.2 Trigger exposure causes the molecules in your immune system to work together to create swelling in your airways. Biologics attach to these molecules and prevent them from causing inflammation and symptoms.3
Different sets of molecules are responsible for certain types of immune reactions. Doctors have discovered which molecules are most active in the immune systems of people with moderate-to-severe asthma. This means that your health care provider might be able to prescribe a biologic that specifically treats your asthma symptoms. This is known as personalized medicine.
Your doctor will decide if asthma biologics would be helpful for you by looking at your symptoms, the type of medicine you are already taking, and blood test results. The two of you will discuss this information and decide on a treatment plan together. A member of your health care team may also reach out to you to review the treatment and answer any questions you might have.
How Do I Take a Biologic?
Most biologics can be taken at home or given in a doctor’s office every one to four weeks. They are given through an injection (shot) or intravenously (through a vein).
How Do FDA-Approved Biologics Target Specific Immune Molecules?
Each biologic affects your immune system in a different way. This section explains how the six FDA-approved biologic treatments target specific immune molecules.
NUCALA (mepolizumab) and CINQAIR (reslizumab) treat eosinophilic [EE-oh-sin-o-FILL-ick] asthma by targeting a molecule called interleukin-5 [enter-LOO-kin-5]. Interleukin-5 is also known as IL-5.4,5,6
FASENRA (benralizumab) targets the IL-5 receptor, where IL-5 binds, and so is similar to NUCALA and CINQAIR in terms of inhibiting the effects of IL-5 in the body.
If you have eosinophilic asthma, IL-5 molecules activate eosinophils (white blood cells) in your bone marrow and move them to your airways, where they cause swelling. This process is called the IL-5 pathway.
NUCALA and CINQAIR interrupt this pathway by blocking IL-5 molecules from communicating with eosinophils, while FASENRA prevents IL-5 from binding the receptor for IL-5 on eosinophils. This prevents eosinophils from moving to your airways and causing swelling.7
DUPIXENT (dupilumab) treats eosinophilic and oral corticosteroid (OCS)-dependent asthma by targeting the receptor (IL-4 receptor alpha chain) for molecules called interleukins 4 and 13 (IL-4 and IL-13).8,9 These are types of proteins produced by the immune system that play a role in asthma.
When you are exposed to an asthma trigger, your body produces IL-4 and IL-13 in response. These proteins cause inflammation in the airways, which makes it harder for you to breathe. They also cause an increase in mucus, which further blocks your airways.
DUPIXENT blocks IL-4 and IL-13 proteins from sending those signals. This helps reduce inflammation and mucus in the airways.9
XOLAIR (omalizumab) treats allergic asthma by targeting an antibody called immunoglobulin E [eh-mu-no-GLAH-byoo-lun-ee] (IgE). IgE triggers a chain reaction that leads to airway tightening.
If you have allergic asthma, your body responds to allergens by producing IgE. IgE tells other cells in your body to release histamine [his-ta-MEEN]. Histamine tightens the muscles around your airways, which makes it hard to breathe. This is part of the IgE pathway.
XOLAIR blocks IgE from communicating with cells that contain histamine. This prevents those cells from releasing histamine and tightening your airways.10
TEZSPIRE (tezepelumab-ekko) treats both allergic and eosinophilic asthma by targeting a molecule called thymic stromal lymphopoietin.11 Thymic stromal lymphopoietin is also known as TLSP.12
Exposure to asthma triggers or allergens causes your body to release TLSP. TSLP triggers a series of reactions in the immune system that cause the production of other proteins and cells that contribute to inflammation in the airways. This is all a part of the TLSP pathway.12 The TLSP pathway is present in both allergic and eosinophilic asthma.
TEZSPIRE works by blocking TLSP from communicating with other cells, which prevents many parts of your immune system from reacting to your asthma triggers.
What Are the Different Types of Moderate-to-Severe Asthma?
There are many types of asthma. Moderate-to-severe asthma can generally be categorized by “type 2” inflammation and “non-type-2” inflammation.
Eosinophilic asthma and allergic asthma are caused by type 2 inflammation.
People with eosinophilic asthma have high levels of white blood cells called eosinophils [EE-oh-sin-oh-FILLS]. Everyone has eosinophils in their immune system. You need them to fight infections and attack unwanted bacteria in the body. Normal levels of eosinophils help keep you healthy, but too many can cause swelling in your airways.13
People with allergic asthma have an overly active immune response to common allergens, like dust or pollen. If you have allergic asthma, breathing in an allergen will trigger your immune system to produce IgE antibodies. An antibody is a molecule that helps fight off harmful substances that enter your body. IgE antibodies release chemicals into your bloodstream that tighten the muscles around your airways, which makes it hard to breathe.14
Eosinophilic and allergic asthma can overlap. If you have severe allergic asthma, it is likely you also have high levels of eosinophils.
Non-type-2 asthma is also called non-eosinophilic asthma. Eosinophils are not present in the airway. Different types of white blood cells build up and cause inflammation. Certain lifestyle factors, such as obesity and smoking, can put you at higher risk for this type of asthma. People with this type of asthma usually do not respond to typical asthma treatments.
Six biologics currently are approved by the FDA for moderate-to-severe asthma. Xolair is approved for allergic asthma. Nucala, Fasenra, and Cinqair are approved for eosinophilic asthma. Dupixent may work for type 2 asthma more broadly (allergic and eosinophilic). Tezspire may work for all types of asthma. Tezspire is the only biologic approved for non-type-2 asthma.
How Do I Find Out What Type of Asthma I Have?
Biomarkers can help identify what kind of asthma you have. They are a wide range of measures that show what is going on in your body. These measures include the cells, molecules, or antibodies found in your tissues, blood, mucus, and other body fluids. Examples of asthma biomarkers are IgE antibodies, eosinophils, and fractional exhaled nitric oxide (FeNO).
Your doctor may ask you to give a blood or mucus sample and/or take a breathing test. These tests measure asthma biomarkers. The results will show your doctor what kinds of cells and molecules are part of the chain reaction in your immune system that make up your asthma symptoms. They will also help identify the specific type of asthma you have. You and your doctor will discuss test results and decide on the best treatment plan for you.
Do I Need to Take a Biologic for My Asthma?
Biologics are a type of controller treatment. Your doctor may prescribe one if you have moderate-to-severe asthma that remains uncontrolled even though you use your routine inhaled medicines or pills regularly.
Your asthma may be uncontrolled if:
- You have asthma symptoms more than two times a week
- You wake up at night with asthma symptoms more than two times a month
- You need your reliever inhaler for symptoms more than two times a week
- You need to take oral corticosteroids more than one time a year
- You are not able to do all your usual activities
Biologics are an add-on treatment. This means your doctor may have you take them with your routine asthma controller medicine, like an inhaled corticosteroid. Over time, you may be able to reduce your inhaled corticosteroid use or stop taking it altogether. Asthma biologics do not replace quick-relief medicines, such as albuterol.
What Are the Benefits of Biologics?
There are many potential benefits of using a biologic to treat your asthma. They include:
- Fewer asthma episodes and symptoms
- Fewer asthma-related trips to the hospital or emergency room
- Decreased use of oral corticosteroids (like prednisone)
- Lower dosage of other controller medicines (if recommended by your doctor)
- Improved lung function
Do Biologics Have Potential Risks or Side Effects?
The FDA has approved six asthma biologics. This means that these treatments are safe and work well for most people. Even though biologics are typically harmless, side effects are still possible. This is true for all medicines and treatments.
Common side effects of biologics include:
- Headache
- Reaction where the biologic is injected
- Sore throat
- Tiredness (fatigue)
- Joint pain
- Skin rash
In rare cases, biologics can also cause a serious allergic reaction called anaphylaxis.
Certain treatments, like Xolair, have a black box warning. This is a safety warning from the FDA. It means you need to be aware of the drug’s side effects and special instructions for safe use. It is important to speak with your doctor before, during, and after starting Xolair or any other new medicine or treatment.
How Long Does It Take for a Biologic to Work?
Biologics work differently for everyone and may not take effect right away. It might be several weeks before you start to notice your asthma is getting better. Keep taking your biologic and any other asthma medicine you have been prescribed until your doctor says it is safe to stop.
Will My Insurance Cover Biologic Treatments?
Many insurance companies will pay for part of a biologic treatment. You will be responsible for paying whatever amount they do not cover.
Most insurance companies consider biologics to be a specialty treatment and may fill it through a specialty pharmacy. This means you may have to have your insurance company preapprove it and you will not be able to get it from your local pharmacy. Biologics can be more expensive than other controller medicines. Your insurance company will have more information on the purchase price and which asthma medicines are covered by your plan.
The drug companies that make biologics may also put information about costs and coverage on their websites. Many of them have patient assistance programs for people without insurance and people whose insurance does not cover asthma biologics. These programs may also help pay copays for people who have insurance that doesn’t fully cover the cost.
Medical Review: November 2023 by Mitchell Grayson, MD
Closed
References
- Center for Biologics Evaluation and Research (CBER). (2019). What are “biologics” questions and answers. U.S. Food & Drug Administration. https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/what-are-biologics-questions-and-answers
- McGregor, M. C., Krings, J. G., Nair, P., & Castro, M. (2019). Role of biologics in asthma. American Journal of Respiratory and Critical Care Medicine, 199(4), 433–445. https://doi.org/10.1164/rccm.201810-1944ci
- Busse, W. W. (2019). Biological treatments for severe asthma: A major advance in asthma care. Allergology International, 68(2). https://doi.org/10.1016/j.alit.2019.01.004
- FASENRA. (2023). About FASENRA. AstraZeneca. https://www.fasenra.com/eosinophilic-asthma-treatment.html
- NUCALA. (2022). How does NUCALA work? GlaxoSmithKline. https://www.nucala.com/severe-asthma/about-nucala/how-nucala-works
- CINQAIR. (2021). How CINQAIR works. Teva Respiratory. https://www.cinqair.com/about-cinqair#how-cinqair-works
- Ghassemian, A., Park, J. J., Tsoulis, M. W., & Kim, H. (2021). Targeting the IL-5 pathway in eosinophilic asthma: A comparison of mepolizumab to benralizumab in the reduction of peripheral eosinophil counts. Allergy, Asthma & Clinical Immunology, 17(1). https://doi.org/10.1186/s13223-020-00507-0
- DUPIXENT. (2023). How DUPIXENT® (dupilumab) works. Sanofi and Regeneron Pharmaceuticals. https://www.dupixent.com/atopicdermatitis/about-dupixent/how-dupixent-works
- Pelaia, C., Heffler, E., Crimi, C., Maglio, A., Vatrella, A., Pelaia, G., & Canonica, G. W. (2022). Interleukins 4 and 13 in Asthma: Key Pathophysiologic Cytokines and Druggable Molecular Targets. Frontiers in Pharmacology, 13. https://doi.org/10.3389/fphar.2022.851940
- XOLAIR. (2023). How XOLAIR may work to treat allergic asthma. Genentech USA and Novartis Pharmaceuticals. https://www.xolair.com/allergic-asthma/why-consider-xolair/how-xolair-may-work.html
- TEZSPIRE. (2023). About Tezspire. Amgen and AstraZeneca. https://www.tezspire.com/is-tezspire-right-for-me/results.html
- West, E. E., Kashyap, M., & Leonard, W. J. (2012). TSLP: A key regulator of asthma pathogenesis. Drug Discovery Today: Disease Mechanisms, 9(3-4), e83–e88. https://doi.org/10.1016/j.ddmec.2012.09.003
- Asthma Canada. (2023). Biologic treatment for severe asthma. Asthma Canada. https://asthma.ca/get-help/severe-asthma/biologics/
- Froidure, A., Mouthuy, J., Durham, S. R., Chanez, P., Sibille, Y., & Pilette, C. (2015). Asthma phenotypes and IgE responses. European Respiratory Journal, 47(1), 304–319. https://doi.org/10.1183/13993003.01824-2014